
Some of the key risk factors that cause colorectal cancer includes family history (of the disease) as well as old age, while obesity, excessive alcohol use, indolence and cigarette smoking are not ignored either. Alongside, people with history of inflammatory bowel disease such as ulcerative colitis and Crohn’s disease reportedly run higher risk of having colorectal cancer than those without such conditions.
Screening tests for early diagnosis of colorectal cancer often prevent the spread of cancer Quite a few screening tests have since been developed to help gastroenterologists such as Dr. V.K. Rai, M.D and others to detect colorectal cancer early, when the disease becomes more treatable. What’s more, some tests that detect or identify the adenomas are known to prevent the development of cancer through proper medical intervention. So, the screening procedure may also be considered as a form of cancer prevention method in most cases.
The following tests and/or investigative procedures are recommended for diagnosing colorectal cancer.
High-sensitivity Fecal Occult Blood Test (FOBT) – Both polyps and colorectal cancers can bleed, while FOBT checks for tiny amounts of blood in feces (stool) that cannot be seen visually. (Blood in stool may also indicate the presence of conditions that are not cancerous, such as hemorrhoids.
As of now, two types of FOBT are approved by the FDA for screening colorectal cancer: guaiac FOBT (gFOBT) and the fecal immunochemical (or immunohistochemical) test (FIT or iFOBT). With both types of FOBT, stool samples collected by the patient with the help of a kit are sent to the gastroenterologist.
Studies, however, have revealed that guaiac FOBT, when performed every 1 to 2 years in people aged 50 to 80 years, it can help reduce the number of deaths (due to colorectal cancer) by 15 to 33%. When FOBT is the only type of colorectal cancer screening test recommended, experts usually suggest yearly testing.
Stool DNA test (FIT-DNA) is the only stool DNA test that has been approved by the FDA, while Cologuard® is a multitarget test that detects tiny amounts of blood in stool (with an immunochemical test similar to FIT) as well as nine DNA biomarkers in three genes that have been found in colorectal cancer and precancerous advanced adenomas.
The DNA comes from cells in the lining of the colon and rectum that are shed and collect in stool as it passes through the large intestine and rectum. As with both types of FOBT, the stool sample for the FIT-DNA test is collected by the patient using a kit and the sample is mailed to a laboratory for testing. A computer program analyzes the results of the two tests (blood and DNA biomarkers) and provides a finding of negative or positive. People who have a positive finding with this test are advised to have a colonoscopy. In one study comprising people who were at average risk of developing colon cancer but had no symptoms of colon problems, this test detected more cancers and adenomas than the FIT test (that is, it was more sensitive). However, the FIT-DNA test also was more likely to identify an abnormality when none was actually present (that is, it had more false-positive results).
In this test, the rectum and sigmoid colon are examined using a sigmoidoscope, which is a flexible lighted tube with a lens for viewing and a tool for removing tissue. This instrument is inserted through the anus into the rectum and sigmoid colon as air (or carbon dioxide) is pumped into the colon to expand it so the gastroenterologist may become able to view the colon lining more effectively. During sigmoidoscopy, abnormal growths in the rectum and sigmoid colon can be removed for analysis (biopsied). The lower colon must be cleared of stool before sigmoidoscopy, but the preparation is less extensive than that required for colonoscopy. People are usually not sedated for this test.
Studies, however, have shown that people who have regular screening with sigmoidoscopy after age 50 years have a 60 to 70% lower risk of death due to cancer of the rectum and lower colon than people who do not have screening. One randomized controlled clinical trial found that even just one sigmoidoscopy screening between 55 and 64 years of age can substantially reduce colorectal cancer incidence as well as mortality. Experts generally recommend sigmoidoscopy every 5 years with or without gFOBT or FIT every 3 years for people at average risk who have had negative test results.
In this test, the rectum and entire colon are examined by using a colonoscope, which is a flexible lighted tube with a lens for viewing and a tool for removing tissue. Like the shorter sigmoidoscope, the colonoscope is inserted through the anus into the rectum and the colon as air (or carbon dioxide) is pumped into the colon to expand it so the doctor can see the colon lining more clearly. During colonoscopy, any abnormal growths in the colon and the rectum can be removed, including growths in the upper parts of the colon that are not reached by sigmoidoscopy. However, a thorough cleansing of the entire colon is necessary before this test. Most patients receive some form of sedation during the test.
Studies reveal that colonoscopy reduces deaths from colorectal cancer by about 60 to 70%. Additional studies are currently being done to better evaluate how effective colonoscopy screening methods are. Experts recommend colonoscopy every 10 years for people at average risk as long as their test results are negative.
This screening method, often called computed tomographic (CT) colonography, uses special x-ray equipment (a CT scanner) to create a series of pictures of the colon and the rectum from outside the body. A computer then assembles these pictures into detailed images that can show polyps and other abnormalities. Virtual colonoscopy is less invasive than standard colonoscopy and does not require sedation. As with standard colonoscopy, a thorough cleansing of the colon is necessary before this test, and air (or carbon dioxide) is pumped into the colon to expand it for better viewing of the colon’s lining. The accuracy of virtual colonoscopy is similar to that of standard colonoscopy, and virtual colonoscopy has a lower risk of complications. However, if polyps or other abnormal growths are found during a virtual colonoscopy, a standard colonoscopy is usually performed to remove them.
Whether virtual colonoscopy can help reduce deaths from colorectal cancer is not yet known, and Medicare and some other health insurance companies currently do not pay for the costs of this procedure. Studies are ongoing to compare virtual colonoscopy with other screening methods.
Apart from the above investigative tests in regard to colorectal cancer, some of the following procedures are also adopted in many cases.
This test, also called DCBE, is another method of visualizing the colon from outside the body. In DCBE, a series of x-ray images of the entire colon and rectum is taken after the patient is given an enema with a barium solution. The barium helps to outline the colon and the rectum on the images. DCBE is rarely used for screening because it is less sensitive than colonoscopy in detecting small polyps and cancers. However, it may be used for people who cannot undergo standard colonoscopy—for example, because they are at particular risk for complications.
Gastroenterologists often perform a single-specimen guaiac FOBT on a stool sample collected during digital rectal examination as part of a routine physical examination. However, this approach has not been shown to be an effective way to screen for colorectal cancer.
Suffering from severe bouts of intestinal pain or having swallowing problems? Go for end
read more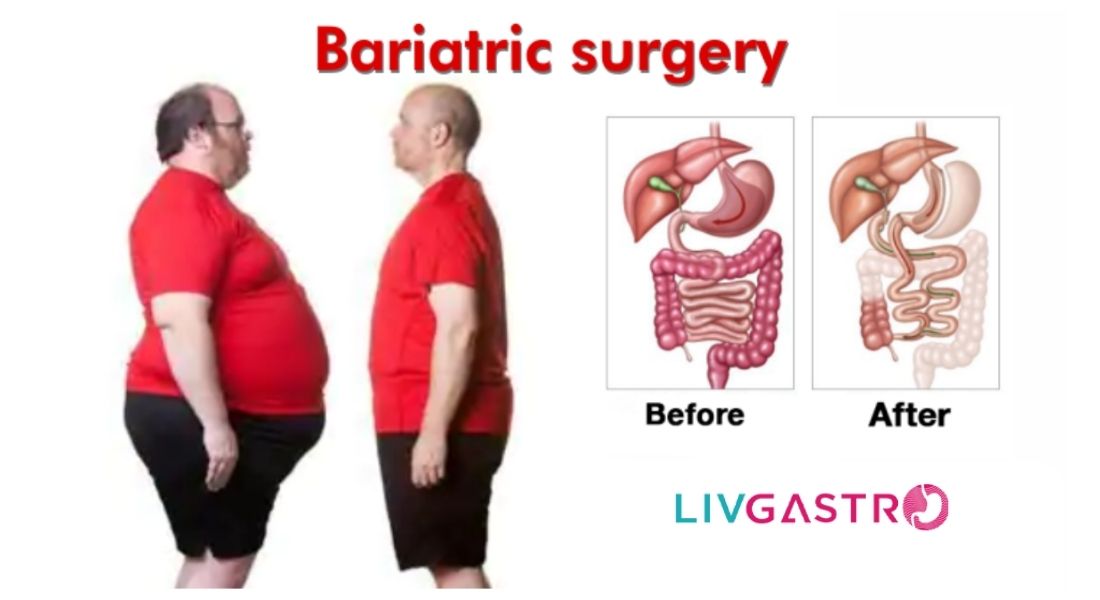
Demystify These Myths Associated with Obesity Treatment Did you know that a staggering
read more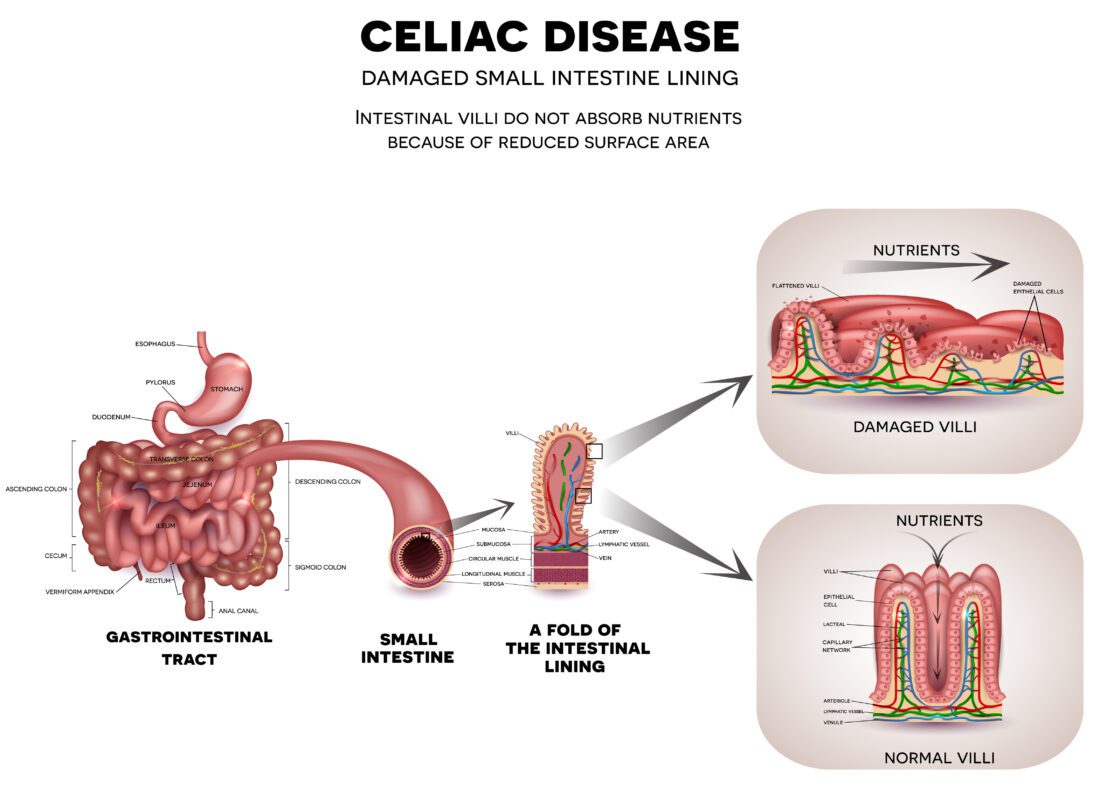
Celiac disease: Cause, Symptoms, Diagnosis and Treatment Why do you think so many people
read more